Abstract
Background and aims
Treatment of relapsed/refractory myeloma (RRMM) remains a challenge as most approved and commonly accessible doublet treatments induce responses (≥PR) in less than half of patients. The combination of the classical alkylator cyclophosphamide with thalidomide (CTD) or lenalidomide (CRD) is standard of care in early lines of therapy in the UK and elsewhere. Data on the clinical value of cyclophosphamide and pomalidomide combination therapy in RRMM is currently sparse and lacking for patients that have previously been treated with cyclophosphamide in earlier lines of therapy.
Material and Methods
MUKseven is a randomised phase II study for RRMM patients comparing cyclophosphamide (500 mg po d1, 8, 15), pomalidomide (4 mg days 1-21) and dexamethasone (40 mg; if ≥75 years 20 mg; d1, 8, 15, 21) (CPd) versus standard pomalidomide and dex (Pd). Patients with ≥2 prior lines of therapy were randomised 1:1 to receive either CPd or Pd and treated until progression. The primary endpoint of the study is PFS, secondary endpoints include response, OS and safety and toxicity. Patients underwent bone marrow sampling and peripheral blood collection at baseline, on treatment and at relapse to correlate outcomes with disease biology. The original sample size was 250 patients but due to approval of pomalidomide by the UK funding agency NICE mid-recruitment, and resulting low enrolment rates, a decision was made to close the trial early.
Results
In total 102 evaluable RRMM patients were randomised between March 2016 and February 2018, 51 each to CPd and Pd treatment arms that were comparable regarding age, gender, ISS and ECOG performance status. Patients had received a median of 3 prior lines of therapy (range 2-8); all had been treated with proteasome inhibitors and lenalidomide and 94% of patients had received cyclophosphamide as part of earlier lines of therapy. Trial entry criteria were permissive and allowed ongoing red cell, platelet and growth factor support to reflect real-world practice in RRMM - 11% of patients required platelet and 16% G-CSF support before starting trial therapy.
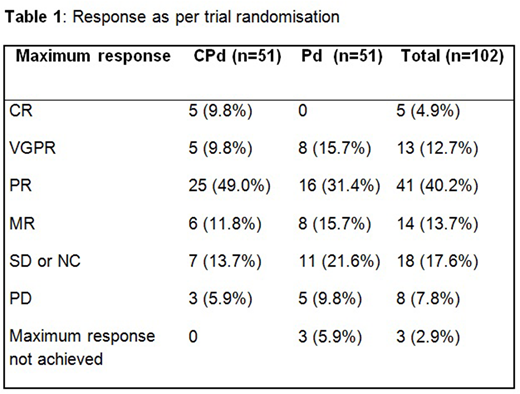
Treatment with CPd was associated with a significantly higher response rate (≥PR) of 68.6% (95% CI: 54.1 - 80.9%) compared to 47.1% (CI: 32.9 - 61.5%) for Pd (P=0.018). Five patients (9.8%) on CPd treatment reached CR vs. none with Pd therapy (Table 1). PFS data is currently maturing and will be presented at the conference. At the time of abstract submission 20 patients were still on trial treatment and 22.5% of evaluable patients had received trial therapy for 12 months or longer.
Anaemia, fatigue and infection of any grade were common side effects and similar in frequency between treatment arms, whereas higher grade cytopenias were more frequent with CPd than Pd. More patients experienced at least one SAE with CPd treatment (44 patients) than with Pd (36 patients). Over 80% of SAEs suspected to be related to study drug for CPd and Pd arms were infections or cytopenias requiring admission for iv therapy. Five patients on the CPd arm and 4 patients on Pd discontinued therapy due to toxicity.
Site and patient adherence to central bone marrow and peripheral blood collection was high with 92% of baseline samples, 87% at C1D14, 87% at C4D14 and 43% of samples at relapse received by central laboratories. High risk genetic lesions gain(1q), del(17p) and adverse translocations t(4;14), t(14;16) and t(14;20) were profiled, amongst other changes, using molecular assays (digital MLPA, TaqMan). Of the 66 patients for whom complete results were available at the point of abstract submission, 48.5% were found to have 1 high risk lesion and 13.6% ≥2 high risk lesions ("double-hit"). The best response achieved in patients with double hit tumours was PR in 1 out of 9 evaluable patients. Further genetic profiling and related exploratory analyses are ongoing.
Discussion
The combination of cyclophosphamide, pomalidomide and dexamethasone significantly increases response rates and depth of response compared to pomalidomide and dexamethasone in RRMM patients, even those that have already been exposed to cyclophosphamide combination therapy in previous lines of therapy. Primary endpoint PFS data for CPd vs. Pd will be presented at the conference. Analyses of outcomes in the context of molecular profiles are ongoing and will be presented at the conference.
Pawlyn:Celgene Corporation: Consultancy, Honoraria, Other: Travel support; Takeda Oncology: Consultancy, Other: Travel support; Amgen: Consultancy, Honoraria, Other: Travel Support; Janssen: Honoraria, Other: Travel support. Garg:Amgen: Honoraria, Other: Travel Support; Novartis: Other: travel support, Research Funding; Janssen: Honoraria; Takeda: Other: Travel Grant. Boyd:Novartis: Consultancy, Honoraria; Janssen: Honoraria, Other: Travel and Accommodation expenses; Celgene: Consultancy, Honoraria, Other: Advisory role. Cook:Bristol-Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Glycomimetics: Consultancy, Honoraria; Celgene Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Honoraria; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau. Kaiser:Amgen: Consultancy, Honoraria; Takeda: Consultancy, Other: travel support; Bristol-Myers Squibb: Consultancy, Other: travel support; Janssen: Consultancy, Honoraria; Chugai: Consultancy; Celgene: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal